Post by Admin on Dec 19, 2021 9:07:43 GMT -7

Organic materials become fossils the same way
geodes and other underground voids are filled.
Ground waters and electromagnetic currents collect various ions and exchange them, or deposit them in empty spaces. "pH" (the power of hydrogen) and the Electromagnetic Force controls the process.
The Electromotive Force Series (EMF series) is a metal's ranking
in respect to inherent reactivity.
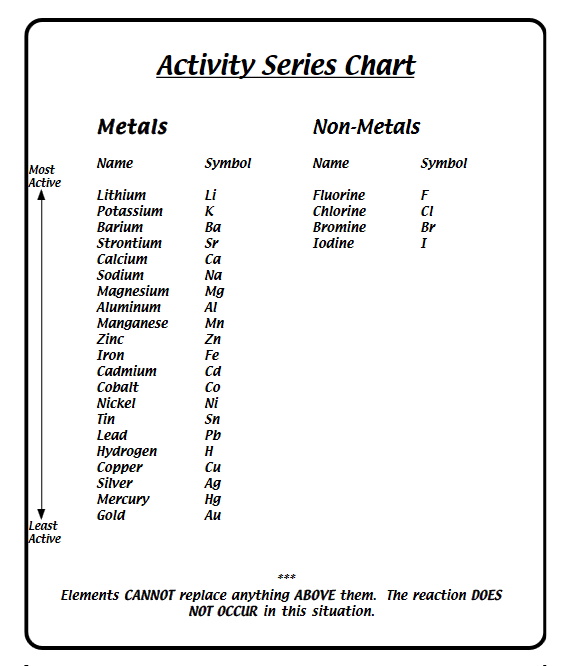
The metals located at the top of the series are considered the most noble, with the highest level of positive electrochemical potential. The metal that can be found at the bottom is the most active and contains the highest amount of negative electrochemical potential.
This series is helpful in determining the tendency of a metal to release energy and corrode.
Single-replacement reactions only occur when the element that is doing the replacing is more reactive than the element that is being replaced. Therefore, it is useful to have a list of elements in order of their relative reactivity. The activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series.
Elements react differently in different circumstances.

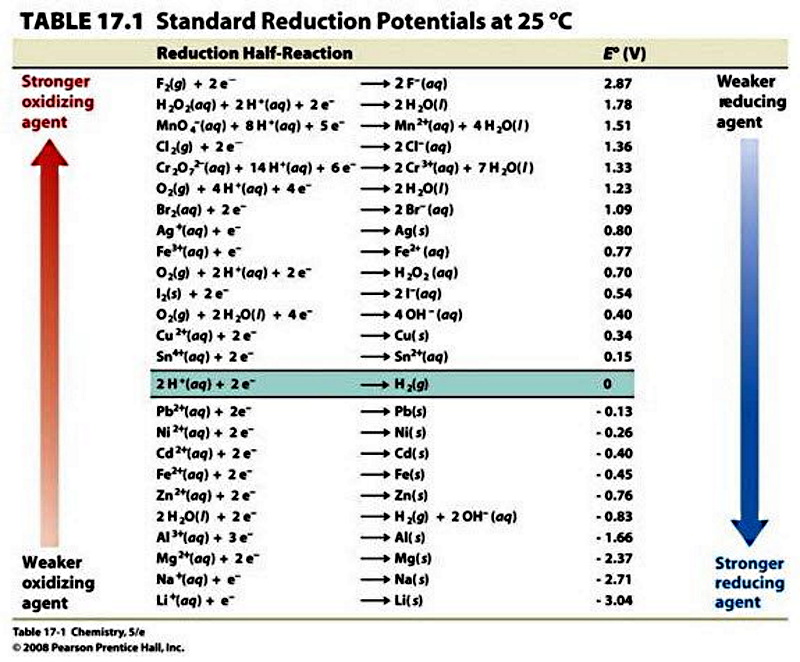
Oxidation vs. Reduction
Reduction and oxidation occur simultaneously in a chemical reaction called a reduction-oxidation or redox reaction.
The oxidized ion loses electrons, while the reduced ion gains electrons.
Despite the name, oxygen need not be present in an oxidation reaction.
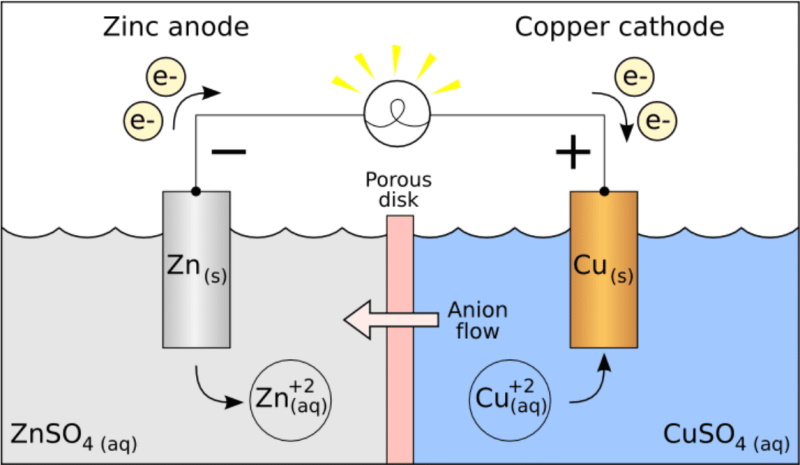
Because of these reactions Natural Batteries are in operation all over.
what was being petrified: Animals

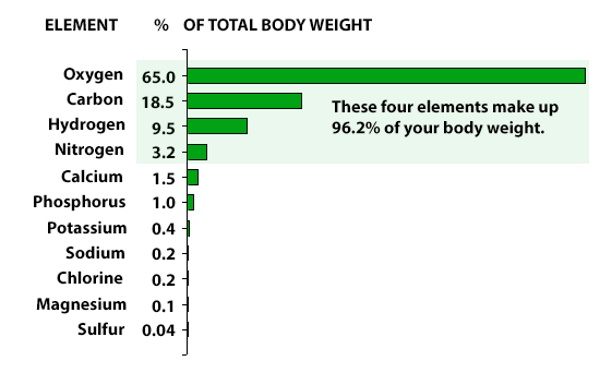
Mostly Bone, teeth, antler and horn, are important elemental storage sites in animals. These tissues contain necessary elements, both major, such as calcium (Ca), phosphorus (P), magnesium (Mg) and sulphur (S), and trace elements, such as iron (Fe), zinc (Zn), manganese (Mn) and cadmium (Cd).

Plants and OrganicMaterial. ~ 95% Water!
www.cliffsnotes.com/study-guides/biology/plant-biology/mineral-nutrition/essential-elements
Over 95 percent of the dry weight of a flowering plant is made up of three elements—carbon, hydrogen, and oxygen—taken from the air and water. The remaining 5 percent of the dry weight comes from chemicals absorbed from the soil. Roots absorb the chemicals present in their surroundings, but only 14 of the elements absorbed are necessary for plant growth.
www.clemson.edu/public/regulatory/ag-srvc-lab/soil-testing/pdf/plant-nutrient-levels.pdf
There are sixteen (16) elements that have been established as essential for the optimal
growth of chlorophyll-containing plants. These elements have been divided into two
groups, nine (9) as major elements [carbon (C), hydrogen (H), oxygen (O), nitrogen (N),
phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S)], since they
are found in relatively high (>1.00%) concentrations in the plant, and seven (7) elements as
micronutrients [boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn),
molybdenum (Mo), and zinc (Zn)], since they are found in relatively small (<0.1%)
concentrations in the plant. Three of the major elements, carbon, hydrogen, and oxygen, are
combined in the process called “photosynthesis” (H2O + CO2 in the presence of light and
chlorophyll = C6H12O6 + O2) forming the carbohydrate structure of the plant, hydrogen (H)
coming from water (H2O) and carbon (C) and oxygen (O) coming from carbon dioxide
(CO2) in the air. These three elements constitute 90 to 95% of the dry matter content of
most plants.
2. Major Mineral Elements
There are 6 essential major mineral plant nutrient elements, [nitrogen (N), phosphorus (P),
potassium (K), calcium (Ca), magnesium (Mg), sulfur (S)], that constitute in total between 5
to 10% of the dry weight of most plants.
Over 95 percent of the dry weight of a flowering plant is made up of three elements—carbon, hydrogen, and oxygen—taken from the air and water. The remaining 5 percent of the dry weight comes from chemicals absorbed from the soil. Roots absorb the chemicals present in their surroundings, but only 14 of the elements absorbed are necessary for plant growth.
www.clemson.edu/public/regulatory/ag-srvc-lab/soil-testing/pdf/plant-nutrient-levels.pdf
There are sixteen (16) elements that have been established as essential for the optimal
growth of chlorophyll-containing plants. These elements have been divided into two
groups, nine (9) as major elements [carbon (C), hydrogen (H), oxygen (O), nitrogen (N),
phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S)], since they
are found in relatively high (>1.00%) concentrations in the plant, and seven (7) elements as
micronutrients [boron (B), chlorine (Cl), copper (Cu), iron (Fe), manganese (Mn),
molybdenum (Mo), and zinc (Zn)], since they are found in relatively small (<0.1%)
concentrations in the plant. Three of the major elements, carbon, hydrogen, and oxygen, are
combined in the process called “photosynthesis” (H2O + CO2 in the presence of light and
chlorophyll = C6H12O6 + O2) forming the carbohydrate structure of the plant, hydrogen (H)
coming from water (H2O) and carbon (C) and oxygen (O) coming from carbon dioxide
(CO2) in the air. These three elements constitute 90 to 95% of the dry matter content of
most plants.
2. Major Mineral Elements
There are 6 essential major mineral plant nutrient elements, [nitrogen (N), phosphorus (P),
potassium (K), calcium (Ca), magnesium (Mg), sulfur (S)], that constitute in total between 5
to 10% of the dry weight of most plants.
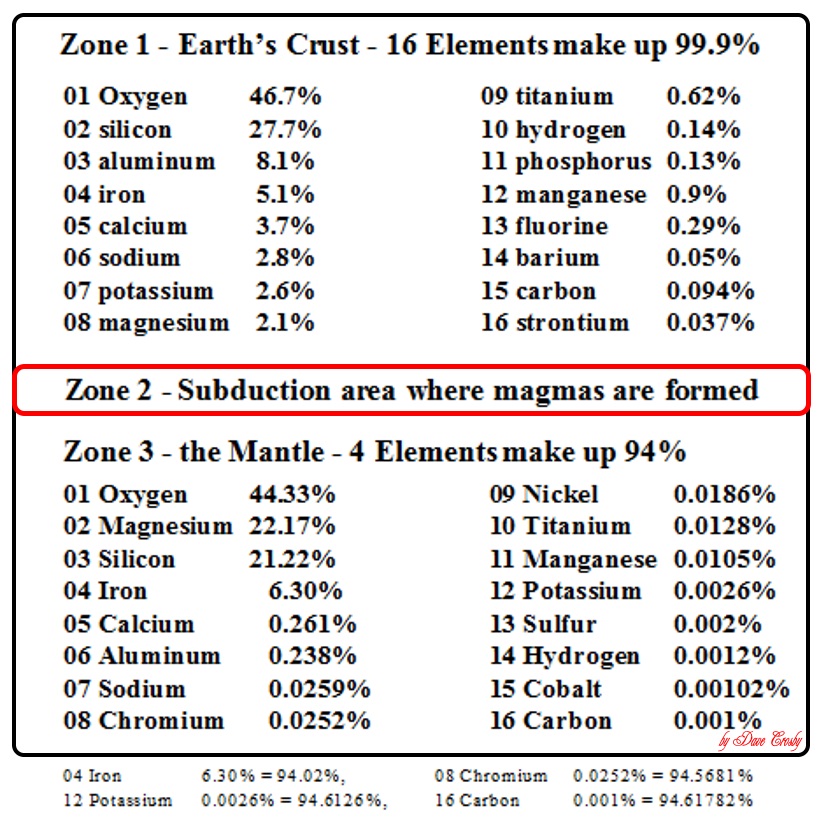
SO, what elements are in the area available for petrifying, and where are they on the charts?
Each area is different because of impacts that occurred there long ago.
Where are the ground waters on the pH scale?
pH

Did you notice SiO2 hasn't been mentioned?
SiO2 can be dissolved, BUT not in quantity, and then only in high pH solutions containing a LOT of magnesium and/or potassium to become "water glass."
And yet we have huge amounts of items petrified with SiO2!
That is because
SiO2 mainly enters solution in a different process.
