Post by Admin on Aug 12, 2022 12:47:01 GMT -7
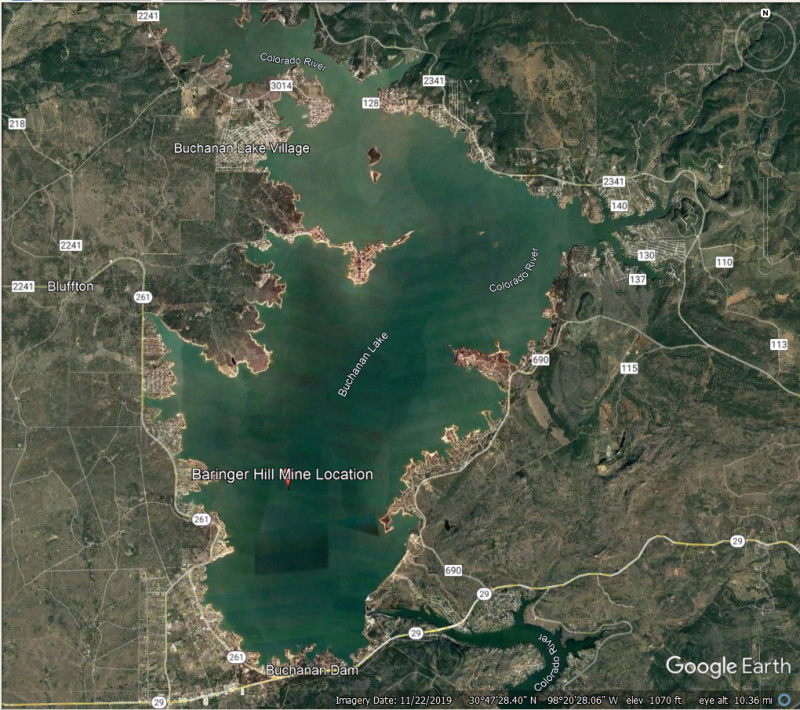
I caught my first fish, a half pound perch, on lake Buchanan when I was 7 years old. Little did I know I was within a mile of a very famous mine site.
first a little about Yttrium.
www.mindat.org/photo-768143.html
In 1787, part-time chemist Carl Axel Arrhenius found a heavy black rock in an old quarry near the Swedish village of Ytterby. Hoping it was an unknown mineral, he named it ytterbite.
Once yttria was found to be a mineral and not an oxide, Martin Heinrich Klaproth renamed it gadolinite in honor of Johan Gadolin.
Hermann Nernst, working for Westinghouse, developed a street lamp that used raw gadolinite as a filament.
It was used in the radio tube industry as a “getter” to burn out the last free oxygen in the tubes.
en.wikipedia.org/wiki/Yttrium
"Yttrium is found in most rare-earth minerals,[9] it is found in some uranium ores, but is never found in the Earth's crust as a free element.[46] About 31 ppm of the Earth's crust is yttrium,[6] making it the 28th most abundant element, 400 times more common than silver.[47] Yttrium is found in soil in concentrations between 10 and 150 ppm (dry weight average of 23 ppm) and in sea water at 9 ppt.[47] Lunar rock samples collected during the American Apollo Project have a relatively high content of yttrium.[42]"
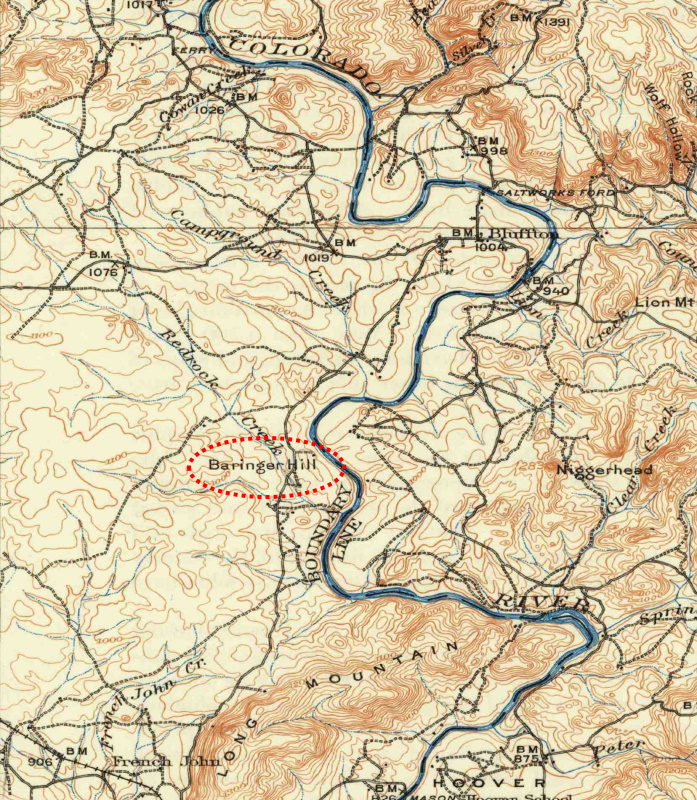
The Saga of Beringer Hill" - 1908 map of Central Texas
the United States Geological Survey once described Baringer Hill as one of the greatest deposits of rare-earth minerals in the world. "the pegmatite was the first place geologists discovered fergusonite, monofergusonite, thorogummite, yttrialite, and nivenite." In all, 47 valuable minerals were identified at the hill.
Baringer Hill lies on the west bank of Colorado River. Paige describes it as follows: "It is a low mound rising about 40 feet above the river which has here a flood plain about one-fourth mile wide. The hill is formed by an irregular pipe or a short dike of pegmatite which has been more resistant to erosion than the surrounding rock, which is a coarse porphyritic granite with feldspar phenocrysts about an inch long and which disintegrates rapidly."
Baringer Hill was named for John Baringer, a carpenter who acquired the land in 1886 in payment for the $50 due for a house he had constructed for a Mr. Wills who was unable to pay. Mr. Wills probably considered it the most worthless part of his farm. What good was a chunk of rocks in the middle of a floodplain?
The following year Baringer discovered that much of the hill consisted of strange heavy, greenish-black rocks that no one coul didentify. In 1887, Professor N.J. Badu of Llano sent ore samples to Philadelphia and New York.
The samples were found to be composed primarily of a radioactive yttria mineral, known as gadolinite, that had previously only been found in small amounts in Russia and Norway. Because they were so rare, yttria minerals were extremely valuable. In 1887, pure yttrium brought $144 an ounce. In comparison, pure gold brought $19 an ounce on the London exchange that same year. Because the minerals from this deposit were so valuable, they were wrapped in tissue paper, packed in iron-bound boxes, and shipped by express at 100 pounds a box.
Discovery of gadolinite at Baringer Hill triggered the interest of the rich and famous. The race for control of the area began when William E. Hidden, a Newark, New Jersey mineralogist with connections to both Thomas Edison and George Westinghouse read a newspaper account about the discovery.
At that time, Edison and Westinghouse were looking for gadolinite to use in the creation of a filament for electric light bulbs but had found no accessible sources of the mineral. Never one to miss an opportunity, Hidden dispatched Dr. William Niven, a Scottish born Texan, to investigate Baringer Hill in 1889. Niven identified forty-seven minerals there, including five previously unknown rare earth elements:fergusonite, monofergusonite, thorogummite, yttrialite, and rarest of all, nivenite.
Years later, Hidden experimented on all the Barringer Hill minerals to ascertain the extent of the "new" form of energy present (e.g., radioactivity). When radiographs were produced by exposing photographic plates to the minerals in the dark, Nivenite produced the most beautiful radiographs because of its pronounced radioactivity.
Hidden then commissioned Niven to buy the land. In 1889, during Niven's second trip, Barringer agreed to sell his land through Hidden and him to the Piedmont Mining Company of London, England (which was owned by Edison.) Depending on the source, the company paid him either $5000 or $10,000 in gold because that was the only way he would take anything in exchange for his land. The gold was picked up in Burnet by Barringer and seventeen year old Tad Casner of Llano County, who was serving as his body guard.
Although Edison experimented with all 47 Barringer Hill minerals, by 1903, the company could find no use for any of them.
Amazingly, during the same year, a German chemist named Hermann Nernst, working for Westinghouse, developed a street lamp that used raw gadolinite as a filament. Later, Nernst would become famous in his own right for discovering the Third Law of Thermodynamics (entropy approaches a minimum at absolute zero), which he won a Nobel prize for in 1920.
Initially, the Nernst lamp had a life of only two hours. Soon, this number was increased to seven hundred hours by another Westinghouse engineer, Marshall Hank. The lamp's design was also modified to accommodate a filament consisting of 25 percent yttria and 75 percent zirconia. These ingredients were made into a paste, squirted into strips, baked, and then cut into the proper lengths. When the mixture was cold, it was nonconductive, but after being heated, it became a conductor that gave off a brilliant light with wavelengths penetrating deep into the infrared.
With its technical problems solved, the Nernst Lamp Company, no doubt a subsidy of Westinghouse, decided to put the lamp into production and bought Baringer Hill through William E. Hidden.
During the winter of 1902-03, the Nernst Lamp Company sent Hidden, himself, to begin excavation.

William E. Hidden next to a 73-lb. mass of Gadolinite in place at Barringer Hill, 1903 (6).
For four months that winter and six months the following year, a dozen or so miners worked full time for the company, including Tad Casner and Barringer, who was probably once again somewhat of a laughing stock for not getting as much money as he could have for the land. Work was slow because the minerals appeared in pockets. The miners struggled to remove ore using picks, shovels, and dynamite to quarry rock from open 40 foot deep pits around the edge of the mound. Despite the difficulty of their task, the men made progress. By 1904, they had blasted away the top of the hill. A 30 foot high quartz pillar, however, was left standing in the middle of the quarry because it contained almost no rare earth minerals.
Hidden and the miners made many curious discoveries about the minerals at Barringer Hill. Masses of purple and green coarsely crystallized fluorite up to 400 pounds were fairly common, as were enormous crystals of feldspar, some over five feet in diameter. From a cavity big enough to hold a horse, the miners removed a 600 pound single smoky quartz crystal which measured 43" X 28" X 15".
Other rock samples were radioactive, such as a seventy-three pound double crystal of gadolinite. Even seemingly common minerals could be radioactive. For example, in a 1905 publication, Hidden mentions that much of the fluorite from Barringer Hill exhibited "brilliant green light when strongly heated and viewed in the dark," and one piece was "self-luminous at night without heat."
Some crystals were taken from areas where the rock matrix contained radiating lines that resembled stars. The stars possibly formed when rare earth elements crystallized from the magma before the quartz in the matrix did, thus causing the quartz to crack as it accommodated itself to the crystals.
Hidden wrote that while removing a mass of mixed zirconium-yttrium-uranium and thorium ore from one of the stars, his hands and face began to burn as if from the effect of strong sunlight. After two or three days of mining, he felt soreness in the parts of his hands and face that had been directly exposed to the minerals.
Because the effects of radiation were not well understood at the turn of the century, Hidden could only speculate as to the cause of his curious symptoms. He reported:
My assistant (Mr. J. Edward Turner) asked me "if these minerals could be poisonous?" As no arsenic was present . . The thought came to me that this action might be the work of a radio-active element and it is offered now more as a suggestion than as a proven fact.
William Hidden was not the only employee Nernst/Westinghouse sent to work at Barringer Hill. In 1903, Marshall Hanks, the engineer who had improved the Nernst Lamp, arrived to run the mining operation. Although Hidden, at least in his writings, seemed to find no fault with the Hill Country or its inhabitants, Hanks did not fare as well. Hanks was unpopular with the miners
because he kept secrets from them. In addition, he had heard many stories about what murderers and scoundrels Texans were.
The miners, therefore, took every opportunity to play pranks on this "green as a gourd" Yankee.
In 1904, Westinghouse had enough gadolinite to suit its needs and decided to recall Hanks. Availing themselves of this last, great opportunity, the miners decided to play one final trick on their boss. At that time, Wells Fargo was in charge of shipping Barringer Hill ore out of Kingsland by train, so the miners brought the Wells Fargo express agent in on the prank. The agent convinced Hanks that the only way he would get out of Texas alive was to mail himself to Pennsylvania in a crate of ore.
After he was loaded up, the miners pretended to search for him in the baggage car. Just as they threatened to shoot at the crates to flush him out, the agent bellowed out, "You fellows better not mess with Wells Fargo! Get out of this baggage car!" Hanks escaped in the crate and always believed the miners had wanted to kill him. Like Hidden, his life does not seem to have been cut short due to exposure to radioactive materials, and he went on to have an illustrious engineering career in Pennsylvania.
For several years, the Nernst Lamp Company continued to annually extract a few hundred pounds of yttria minerals from Barringer Hill. Eventually, however, Nernst ceased operations as newer technologies surpassed the lamp.
From the time mining operations ended until the building of Buchanan Dam flooded the area, Barringer Hill was of interest primarily to mineralogists. One of the best collections of minerals from the area was that of Tillie Badu Moss, Professor Badu's daughter. Among her specimens were pieces of quartz which had been beautifully colored through exposure to radiation.
According to Tillie Badu Moss specimens are to be found in many museums, including the Houston Museum of Natural History and the New York Museum of Natural History. The University of Texas at Austin also possesses a number of rocks from Barringer Hill, including the 600 pound smoky quartz crystal that so impressed William Hidden. For many years, it was
displayed on the Little Campus at UT until renovation began in the 1970's. Like many other exotic Texas mineral specimens, it is now squirreled away as part of the geology department's Barron Collection.
Other crystals found their ways into museum collections across the country. For instance, the Smithsonian Institute has a large, faceted piece of gadolinite that was once part of a neckdrop. Supposedly the radioactivity from this gem led to the premature death of its original owner.
Photos from 1932 -


Thoughts from THE BARINGER HILL, TEXAS, PEGMATITE by Kenneth K. Landes, University of Kansas
The minerals in the Baringer Hill pegmatite were deposited in definite sequence during two distinct phases. The first of these phases was essentially magmatic, while the second was hydrothermal. The space occupied by the minerals forming during the first phase was in most part space previously occupied by the pegmatite magma with replacement of secondary importance, while in the second phase replacement was the dominant process.
First phase. The sequence of events during the first phase is considered to have been as follows:
1.Intrusion of pegmatite magma. This magma contained abundant water and other volatile constituents and was presumably a residual concentrate of the magma which formed the surrounding granite. The effect of the volatiles was largely to decrease viscosity and lower the temperature of crystallization so that large crystals could form.
2.Crystallization of microcline, with the formation of crystal outlines in part.
3.Partial replacement of microcline by albite, producing perthite. This replacement was due to reaction between the microcline and the residual very mobile pegmatite magma. Alling19 calls such perthite deuteric.
4.Crystallization of quartz, forming very large masses toward the center of the pegmatite and replacing the microcline in the peripheral zone in such a manner as to form graphic granite. The evidence for the replacement origin of the graphic granite has already been given.
The crystallization of the quartz completed the solidification of the pegmatite magma through that part of the pegmatite now at or close to the surface.
Second phase. The volatile constituents of the pegmatite magma did not enter into the composition of the minerals formed during the first phase. Consequently, with the crystallization of the pegmatite magma to successively lower depths in the pipe-like intrusive, the residual magma became richer and richer in volatiles, eventually becoming a hydrothermal solution. Some of this solution escaped upward through the pegmatite (the easiest channel of escape) dissolving older minerals and depositing new ones as it did so. Evidences of this hydrothermal activity are the general confinement of the minerals classified in the second phase to a restricted part of the pegmatite (the red rock shoot), their constant association with each other, and the presence of irregular veins and other criteria of replacement which will be described in more detail subsequently. The sequence of mineralization during the second phase was as follows:
1.Deposition of fluorite. This mineral is decidedly veined and otherwise encroached upon by albite.
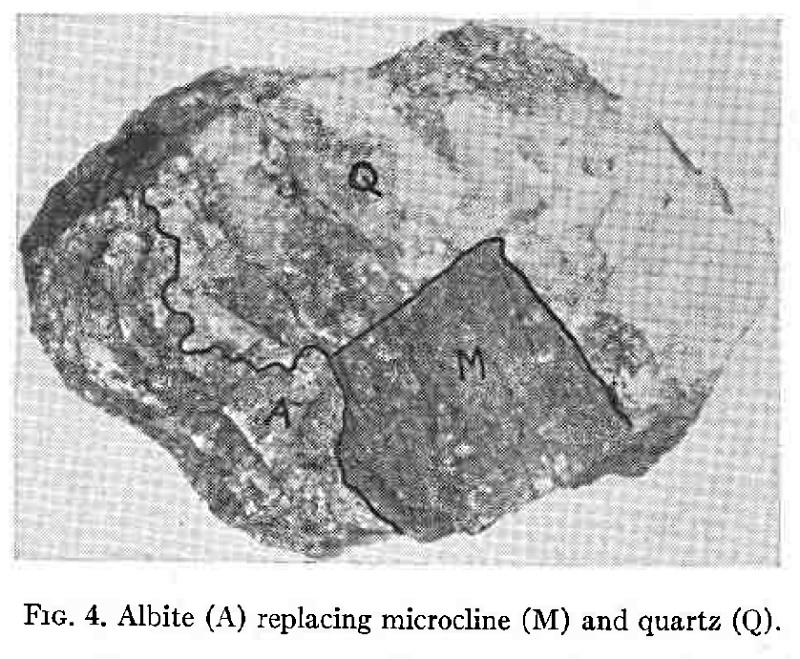
2.Albitization. Subsequent to the deposition of fluorite the ascending solutions deposited great quantities of albite, forming the red rock previously described. Fig. 4 illustrates the replacement of quartz and microcline by albite. As would be expected, the solutions dissolving microcline and depositing albite were unable to dissolve the albite in the original perthite. This explains the presence of older albite in the red rock thin sections. Albite is an invariable associate of the
rare earth minerals. Similar associations have been described by Spence: "In as far as the writer's own observations on the granite pegmatites of eastern Canada are concerned, soda-rich feldspar.... is particularly in evidence around the rare element minerals, allanite, cyrtolite, titanite, monazite, etc. Albitization of original microcline in such situation may, therefore, be presumed to have taken place."20 Albitization was accompanied by cavity formation, due to solution exceeding precipitation in parts of the pegmatite. Various points substantiating the theory that cavities in pegmatites are formed by hydrothermal solution have been given by the writer elsewhere.21 The large cavity already described and smaller cavities noted by Hidden and Mackintosh22 were formed during this stage. Some became lined with albite crystals while later solutions deposited quartz crystals in others.
3.Deposition of quartz (second generation) both in the red rock (giving portions of this rock a graphic texture) and as euhedral smoky and amethystine crystals in cavities.
4.Deposition of rare earth and accompanying minerals. The hydrothermal phase was completed with the deposition of the rare earth minerals and magnetite, biotite, ilmenite and other minerals. Undoubtedly these minerals likewise were precipitated in definite sequence, but they are so rarely found in juxtaposition that it was impossible for the writer to work out the details of the sequence. As a general rule the minerals in this final group are confined to the red rock, as in
the case of the magnetite veins, although in a few places the depositing solutions worked out into the normal pegmatite and there precipitated. Thus Hess noted the presence of lithium mica in cracks in the quartz.23 Likewise some of the rare earth minerals were deposited in massive quartz. Where this occurs the surrounding quartz is radially shattered.
According to Hidden these "stars" were sometimes 8 to 10 feet across. They always had thorium, uranium or zirconium minerals at the center and thus acted as a guide to ore.24 In the writer's opinion the best explanation for such stars has been given by Hess and Wells: "The radial cracks surrounding minerals and pegmatites are connected with the growth of minerals.... The growth took place faster than the older mineral is replaced, breaking the rock radially."25
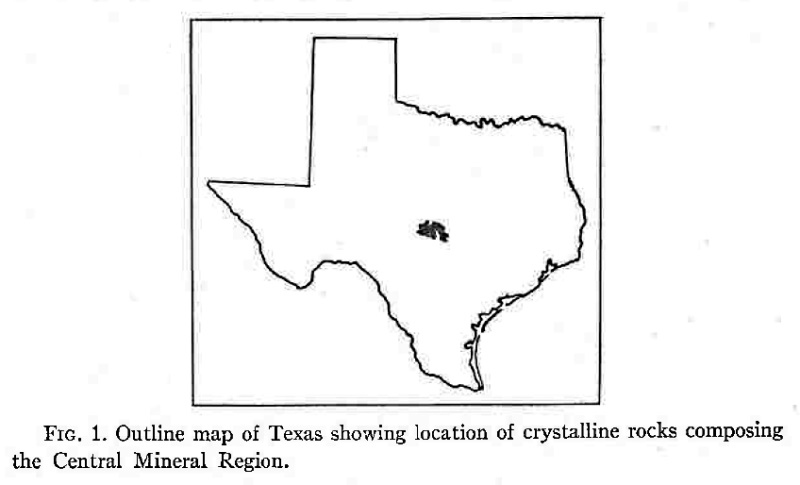
The Baringer Hill pegmatite is intruded into Algonkian (?) granite, which in turn is intruded into the Valley Spring and Packsaddle metamorphic formations. A number of other pegmatites beside that at Baringer Hill occur in the Central Mineral Region, some of which likewise contain rare earth minerals, but no others have been developed to any extent.
The Central Mineral Region is an older name for the area around Llano, Texas. This name comes from the rich mineral deposits found only in this portion of the state. Semi-precious gems and other economically important rocks are found beneath the ground here. These minerals come from the area’s long history of geological uplift and erosion. The diversity of the minerals found here and their amounts have given this area its name of the Central Mineral Region.
Minerals to Find There
Central Mineral Region may contain topaz and other semi precious stones
Photo: Flickr/James St. John
Minerals found in this area include precious metals such as gold and copper and not-so-precious but important materials like talc and iron. While gold was only ever found in limited quantities at the Heath mine in the area, you can still find some topaz on private ranches in the Hill Country in Mason County. Perhaps the most commonly known rock associated with the area is pink granite, much of which is Town Mountain Granite, near Marble Falls. Before mining took it over, Town Mountain likely resembled the more popular granite dome of Enchanted Rock.
You may be more familiar with the modern name of the Central Mineral Region, the Llano Uplift. This name refers to the higher land of the area compared to the rest of the Hill Country. In fact, this region is the only place in the Hill Country to find mountains, or at least what Texans call mountains. Another popular name for this part of the state is the Highland Lakes. Highland refers to the land’s higher elevation, and the lakes refer to the six major bodies of water created along the Colorado River. Boaters and tourists tend to refer to this region as the Highland Lakes rather than the Central Mineral Region or the Llano Uplift.
References
1.Barler, Miles, Early days in Llano, 1905.
2.Fry, Tillie Badu Moss, A history of Llano County, Texas. Thesis, University of Texas at Austin, 1943
3.Geologic Atlas of Texas: Llano Sheet, Bureau of Economic Geology - The University of Texas at Austin, Virgil E. Barnes Edition, 1981.
4.Govin, Charles Thomas, Sedimentation survey, Lake Buchanan Texas. Thesis, University of Texas at Austin, 1973.
5.Hess, F. L., Minerals of the rare-earth metals at Baringer Hill, Llano County, Texas: U.S. Geol. Survey Bull. 340, pp. 286-294, 1908.
6.Hidden, W. E., Some results of late mineral research in Llano County, Tex. Am Jour. Sci., 4th ser., vol. 19, No. 114 - June 1905, pp. 425-433.
7.Highland Lakes north, Buchanan, Inks, L.B.J., and Marble Falls Lakes; contoured depth aerial map for the fisherman, boat.. A.I.D. Associates, 1971.
8.Landes, K. K., The Baringer Hill, Texas, Pegmatite: Amer. Min., vol. 17, pp. 381-390, 1932.
9.Llano County centennial, Llano County Centennial Association, 1956.
10.Oatman, Wilburn Jr. and Sarah Oatman Franklin, Llano Gem of the Hill country: Revisited, 1988, Anchor Publishing, San Angelo.
11.Paige, Sidney, Description of the Llano and Burnet quadrangles: U.S. Geol. Survey Geol. Atlas, Llano-Burnet Folio (No. 183), 16 pp., 1912.
12.Rob Reed's Granite Page (http://uts.cc.utexas.edu/~rmr/)- an excellent site covering not only research into the granites near Barringer Hill, but also the geology of the area.
13.Yarbrough, C.L., Canyon of the Eagles: A History of Lake Buchanan and Official Guide to the Vanishing Texas River Cruise., 1989.
14.Lower Colorado River Authority, Lake Buchanan Fishing, Recreation, and Tourism Map, (Contours from August, 1991 LCRA Hydrographic Survey), LCRA, 1993.
15. Kenneth Knight Landes The Barringer Hill, Texas, pegmatite, American Mineralogist (August 1932) 17 (8): 381-390
URL: www.minsocam.org/ammin/AM17/AM17_381.pdf
16. Yttrium en.wikipedia.org/wiki/Yttrium
17. Barringer Hill en.wikipedia.org/wiki/Barringer_Hill