Post by 1dave on Nov 10, 2020 8:08:45 GMT -7
<<< Back to Understanding SiO2 <<<

Silica gel is an amorphous and porous form of silicon dioxide, consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum.
The best explanation of the gels I have found is in -
"Hydration of Silica and Its Role in the Formation of Quartz Veins – Part 1" by John Elliston.
The solubility of quartz is a function of temperature, pressure, ionic strength, pH, and the presence of complexing ions;
Silica disperses in water to form three classes of solutes (Iler2, 1979, pp 172-248; Stöber4, 1966, pp 161-163): -
1. simple monomeric complexes such as Si(OH)4aq.
2. oligomers or polynuclear complexes such as Si4O6(OH)62- which represent condensed chains of Si(OH)4 tetrahedra (Figure 1). These chains appear to grow to a maximum of about six Si(OH)4 tetrahedra.
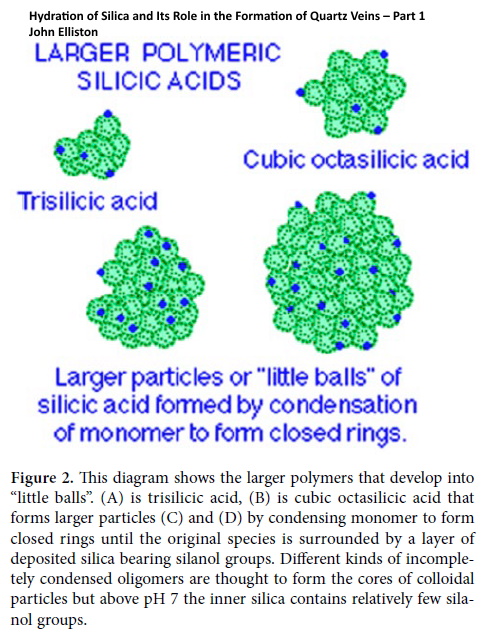
3. poly silicic acid where very long chains are produced with cross-linking to yield partly condensed three dimensional networks. These polymeric species are ‘colloidal’ in that they are frequently loose spherical aggregates of the size that produces scattering of light or gives a “milky” appearance (Figure 2).
The higher polymeric forms of silicic acid tend to occur in solution, re-adsorbed on surfaces, or in suspension as colloidal sol particles, depending on hydrolysis and ionization.
Surface charge
All solid and crystal surfaces are charged including very small gelatinous natural sediment particles such as clay and the minute globular particles of polymeric silica.
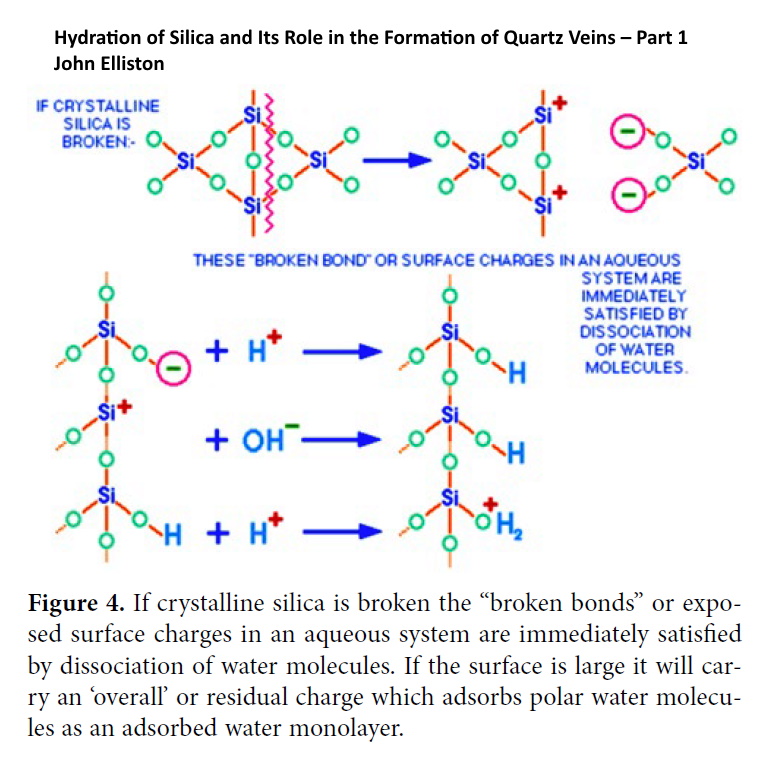
Figure 3 is a TEM image (from Stumm5, 1992) that shows the “bumps” due to charge sites where additional molecules would join the surface to result in crystal growth. These electrical charges are arranged in miniscule steps on the crystal surface. The surface reactivity differs according to the way the units of the lattice are exposed at its surface.
If we are to understand why the globular particles or “little balls” of silica interact with each other and form the precipitates, chains, coatings of adsorbed particles, and gel mesh-works as described by Iler2, it is necessary to consider the basic hydrolysis of the mineral water interface. Similar equilibria relate to a great many irregularities but in natural basin sediments clay minerals present really enormous areas of Si-OH terminations to equilibrate with pore fluid sols and solutions. These immense surfaces are fully loaded with adsorbed ions and charged particles. They all carry adsorbed water monolayers and diffuse charge layers in which the co-ions and counter-ions reside in a narrow zone out from the surface (Hemholtz double layers – see Glossary).
Page 60 - "The data from homogenisation of fluid inclusions in vein quartz repeatedly indicate low to moderate temperatures usually less than half that assumed for magma melting. This has even led committed magmatists to abandon the idea of molten silica injection to form quartz veins." Most quartz veins were formed at moderate temperatures !
Silica disperses in water to form three classes of solutes (Iler2, 1979, pp 172-248; Stöber4, 1966, pp 161-163): -
1. simple monomeric complexes such as Si(OH)4aq.
2. oligomers or polynuclear complexes such as Si4O6(OH)62- which represent condensed chains of Si(OH)4 tetrahedra (Figure 1). These chains appear to grow to a maximum of about six Si(OH)4 tetrahedra.
3. poly silicic acid where very long chains are produced with cross-linking to yield partly condensed three dimensional networks. These polymeric species are ‘colloidal’ in that they are frequently loose spherical aggregates of the size that produces scattering of light or gives a “milky” appearance (Figure 2).
The higher polymeric forms of silicic acid tend to occur in solution, re-adsorbed on surfaces, or in suspension as colloidal sol particles, depending on hydrolysis and ionization.
Surface charge
All solid and crystal surfaces are charged including very small gelatinous natural sediment particles such as clay and the minute globular particles of polymeric silica.
Figure 3 is a TEM image (from Stumm5, 1992) that shows the “bumps” due to charge sites where additional molecules would join the surface to result in crystal growth. These electrical charges are arranged in miniscule steps on the crystal surface. The surface reactivity differs according to the way the units of the lattice are exposed at its surface.
If we are to understand why the globular particles or “little balls” of silica interact with each other and form the precipitates, chains, coatings of adsorbed particles, and gel mesh-works as described by Iler2, it is necessary to consider the basic hydrolysis of the mineral water interface. Similar equilibria relate to a great many irregularities but in natural basin sediments clay minerals present really enormous areas of Si-OH terminations to equilibrate with pore fluid sols and solutions. These immense surfaces are fully loaded with adsorbed ions and charged particles. They all carry adsorbed water monolayers and diffuse charge layers in which the co-ions and counter-ions reside in a narrow zone out from the surface (Hemholtz double layers – see Glossary).
Helmholtz double layer: in an aqueous electrolyte solution in the vicinity of a charged surface the aqueous phase is divided into four regions of distinct dielectric behavior. The innermost region) consists of preferentially oriented water molecules in contact with the solid surface and where specific ions are adsorbed without their hydration shells. This is called the inner Helmholtz layer. The region further from the surface (β in Figure 1.7 in Elliston9, 2017) contains both free water molecules and molecules attached to hydrated ions. This is called the outer Helmholtz layer and is defined by the by the closest approach that a fully hydrated charged ion can make to the solid – liquid interface. Further out from the surface the concentration of counter-ions (having a charge of opposite sign to the surface) decreases with increasing distance in the Gouy-Chapman diffuse layer. The outer and inner Helmholtz layers are referred to as the Helmholtz double layer.
Page 60 - "The data from homogenisation of fluid inclusions in vein quartz repeatedly indicate low to moderate temperatures usually less than half that assumed for magma melting. This has even led committed magmatists to abandon the idea of molten silica injection to form quartz veins." Most quartz veins were formed at moderate temperatures !



>>> Forward to Opal >>>